Everybody has seen those commercials for new drugs. Whether they're treating depression, fibromyalgia, or a chronic illness, they all had to go through the same development process; from its original synthesis to the extensive FDA trials, odds are radiolabeling was used at some point.

Laboratories across the world work tirelessly to develop new medicines, manufacture current medications, and further our understanding of science. When medicine is involved, clean room standards must be upheld. This involves maintaining and monitoring the lab's humidity (electronic sensing devices now ensure a plus or minus 1% accuracy), air purity, and ensuring that no cross-contamination occurs.

Laboratory environments vary depending on the work being done. Regardless of the field of study or experimentation being performed, safety is always paramount. Fume hoods are a staple among basic laboratory safety equipment as they can perform multiple functions, the two foremost being air filtration and chemical protection.

Science is an expansive and constantly evolving field. As more discoveries are made, our understanding of the world we live in grows and develops in (occasionally) incomprehensible ways; we learn what benefits us, what harms us, and everything in between. Laboratories are often at the forefront of such revelations and are protected as such.

Radiolabeled compounds are extremely useful in the modern medical industry, and extremely beneficial to society. From drug trials to cancer treatments, the synthesis of carbon 14 production (also known as 14C labeling) can save lives by allowing new drugs to become available nationally.

Radiolabeled compounds rely on the use of radioactive isotopes (also referred to as radioisotopes) for enrichment. Since radiation is historically bad for human beings, extra care must be taken to ensure that no harm comes to either the people involved in developing these radiochemicals or the compounds themselves; after all, if improper handling procedures were common in this country, the U.S. would not be the number one largest national producer of chemical products globally.

Acute kidney injury (AKI), previously known as acute renal failure, kills around 1.7 million people every year. The kidneys produce a rapid buildup of nitrogenous wastes and also decrease urine output; severe complications -- including muscle weakness, paralysis, and heart rhythm problems -- ensue.
 The U.S. has long been a driver of scientific discovery. From cures to vaccines, medical implementation of these advances has been at the forefront of our nation's goals. The finding of radiolabeling applications is one such breakthrough.
The U.S. has long been a driver of scientific discovery. From cures to vaccines, medical implementation of these advances has been at the forefront of our nation's goals. The finding of radiolabeling applications is one such breakthrough.
Radiolabeling (and isotopic labeling) is valuable to the developments of other breakthroughs: C14 radiolabeling (also known as 14C labeling) allows scientists and researchers to track the passage of an isotope -- an atom with a detectable variation in neutron count -- through a reaction, metabolic pathway, or cell. For example, if you wanted to create a new drug to treat cancer, you'd want to be sure that it's going to the region infected with cancer, preferably the cancer cells themselves. These radiolabeled compounds are visible so scientists can know for sure that their drugs are where they're supposed to be.
However, when you're dealing with radioactive materials, even when they're as minuscule as radionuclides, certain rules and regulations need to be put in place. In 1975, the Food and Drug Administration (FDA) approved the use of radioactive drugs as safe, but only if they met certain requirements. The Radioactive Drug Research Committee (RDRC) Program began the same year to "[permit] basic research using radioactive drugs in humans without an Investigational New Drug Application(IND) when the drug is administered under the following conditions": that the research is considered "basic science research" and is done for the purpose of advancing scientific knowledge; the research study is approved by an FDA-approved RDRC; the dose of the radioactive drug is known not to cause any clinically detectable pharmacologic effect in humans; the radiation dose is justified by the quality of the study being undertaken and the importance of the information it seeks to obtain.
In a similar, equally important vein, any scientific research or testing in a laboratory must be held to the GMP standards. Good Manufacturing Practices are vital to preventing contamination that could destroy the efforts made so far, as well as potentially harm others: in 2012, a fungal meningitis outbreak occurred in a pharmacy in the Northeast due to poor manufacturing procedures -- 48 people died because of the shortcuts, lapse in cleanliness, and poor maintenance of the pharmacy.
If we are to continue to make leaps and bounds in the name of science, it's absolutely essential that we follow GMP standards (as well as RDRC codes) to the letter.
 Getting a new drug off the ground takes a lot of work. You have to follow stringent (and very specific) FDA guidelines, exercise top-tier clean room standards (and GMP standards) to prevent contamination and reduce risk, and perform dozens of radiolabeled tests to ensure the drug is going to the right target within the human body. With so many hoops to jump through and factors to keep track of, it's a miracle we've produced the thousands of drugs already on the market! (more…)
Getting a new drug off the ground takes a lot of work. You have to follow stringent (and very specific) FDA guidelines, exercise top-tier clean room standards (and GMP standards) to prevent contamination and reduce risk, and perform dozens of radiolabeled tests to ensure the drug is going to the right target within the human body. With so many hoops to jump through and factors to keep track of, it's a miracle we've produced the thousands of drugs already on the market! (more…)

When it comes to pharmaceuticals, proper storage is absolutely essential. Guidelines and regulations for GMP storage conditions are in place because even if a product is manufactured properly, something can go wrong if the product is stored improperly. So let's explore a few basic guidelines to keep in mind when storing pharmaceuticals.
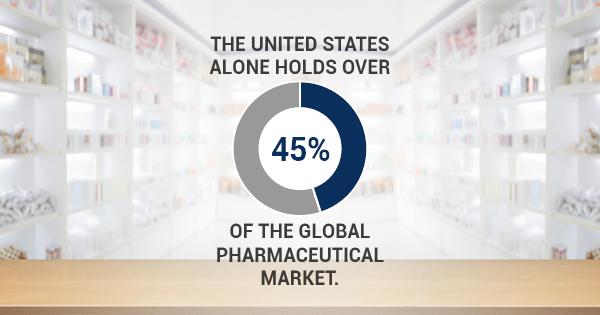
With the U.S. holding more than 45% of the global pharmaceutical market, it's important for laboratories to abide by certain regulations. The regulations in place help to maintain pharmaceutical quality so they're safe for consumers. Good Manufacturing Practice (GMP) regulations give pharmaceutical labs standards they need to meet to ensure their products are both safe and effective. So let's take a closer look at why GMP regulations are so important in today's pharmaceutical labs.
 Carbon is an element that has been used for a variety of scientific purposes over the years, like radiocarbon dating, which was developed around 1946 by Willard F. Libby. But in today's pharmaceutical labs, carbon 14 plays a crucial role in drug development. And because of depleting supplies and increasing prices, carbon 14 production is at an all-time high. But what can carbon 14 be used for and how is it used safely? Let's take a look at a few important things to know about C14 radiolabeling.
Carbon is an element that has been used for a variety of scientific purposes over the years, like radiocarbon dating, which was developed around 1946 by Willard F. Libby. But in today's pharmaceutical labs, carbon 14 plays a crucial role in drug development. And because of depleting supplies and increasing prices, carbon 14 production is at an all-time high. But what can carbon 14 be used for and how is it used safely? Let's take a look at a few important things to know about C14 radiolabeling.
